|
Expand All
|
|
|
|
Plant quarantine is a legislative measure enforced to regulate the introduction of seeds, plants/ planting materials, plant products, soil, living organisms etc. in order to prevent inadvertent introduction of insect pests, nematodes, pathogens (fungi, bacteria and viruses) and weeds harmful to the agriculture/ state/ region, and if introduced, prevent their establishment and further spread. Transboundary movement of plant material carries the risk of entry of the associated pests. NBPGR has been given the responsibility on behalf of the Government of India to carry out quarantine checks on the plants/ planting material meant for research purposes for both public and private sectors. Detailed quarantine examination is carried out for fungi, bacteria and viruses, insects, nematodes and weeds.
1. MANDATE
- Quarantine Processing of Plants/ Planting Material under Transboundary Exchange
- Pest-free Conservation of Indigenously collected Germplasm
- Supportive Research to Develop Techniques for Detection and Salvaging of Germplasm
- Policy Issues on Related Biosecurity Issues
- Human Resource Development
|
|
|
|
Import Quarantine
- A total of 6,29,354 samples of various imported crops were processed for quarantine clearance during 2013-2019 of which 11,698 samples were found infested/ infected with various pests (insects/ mites- 2,881, nematodes- 1,309 fungi/ bacteria- 5,185, viruses- 600, weeds- 1,723).
- Among infected samples 8,466 samples were salvaged and rest were rejected. Samples given prophylactic treatments include, fumigation (39,117), hot water treatment to paddy (52,006), pesticidal dip/spray to vegetative propagules (7,322), and 10% tri-sodium orthophosphate (9,731) to capsicum (4,572), tobacco (1) and tomato (5,158) seeds.
- A total of 5,870 samples of exotic germplasm grown in PEQ greenhouses. Besides, 124-post entry quarantine inspection of ~120000 samples for various crops were conducted at indenter’s sites.
- Total 162 pests economically important pests were intercepted comprising insects (20), fungi (58), bacteria (1), viruses (31), nematodes (9) and weeds (43). Of these 49 pests comprising insects (5), fungi (4), viruses (14), and weeds (26) are not yet reported from India. Nine pests comprising fungi (1), viruses (7) and nematode (1) are not reported on the host in India.
Export Quarantine
- A total of 8,615 indigenous crops meant for export to other countries were processed for quarantine clearance, of which 274 samples were found infested/ infected and 193 samples were salvaged and 63 Phytosanitary Certificates were issued.
Seed Health Testing for Pest-Free Conservation
- A total of 1,14,988 indigenous including 937 cryo-preserved samples were processed for pest-free conservation, of which 11,942 samples were found infected with different pathogens and 981 samples were rejected due to heavy infection.
Supportive Research was conducted on
- Development of LAMP and other PCR based diagnostics for fungal/bacterial pathogens and studies on genetic diversity of fungal pathogens were undertaken;
- RT-PCR protocols for detection of various quarantine viruses developed;
- Feasibility of thermal treatments/ non-edible oils against various storage insect pests and on molecular diagnostics of insects;
- Techniques for detection and salvaging treatments for nematodes along with survey of nematode infected pomegranate orchards in and around Jodhpur and guava orchards of Tamil Nadu undertaken.
Quarantine Clearance of Germplasm under exchange
A total of 6,34,969 samples of various crops comprising nurseries/trial, breeding material including both true seed and vegetative propagules were processed for quarantine clearance, of which 6,29,354 samples were imported and 8,615 were meant for export.
a. Import Quarantine: All the imported samples were examined for detection of associated pest and pathogens viz., insects/mites, plant parasitic nematodes, plant pathogens (fungi, bacteria, viruses) and weed seeds by visual examination followed by specific pest detection techniques.
- A total of 11,843 samples of different crops were found infested/ infected with various pests (insects/ mites- 2,881, nematodes- 1,309 fungi/ bacteria- 5,185, viruses- 600, weeds- 1,868).
- Among infected samples 8,466 samples were salvaged by various techniques and treatments and rest were rejected.
- Samples of some crops were given prophylactic treatments viz., fumigation (39,117), hot water treatment to paddy (52,006), pesticidal dip/spray to vegetative propagules (7,322) which also includes formaldehyde root dip treatment to rooted material against nematode pests, and 10% tri-sodium orthophosphate (9,731) to capsicum (4,572), tobacco (1) and tomato (5,158) seeds.
- A large no of economically imported insects, mites and nematodes, fungi, bacteria and viruses were intercepted. A total of 165 pests comprising insects (20), fungi (58), bacteria (1), viruses (31), nematodes (9) and weeds (43) were intercepted. Of these 49 pests comprising insects (5), fungi (4), viruses (14), and weeds (29) are not yet reported from India (Table 1). Nine pests comprising fungi (1), viruses (7) and nematode (1) are not reported on the host in India (Table 2).
b. Export quarantine: A total of 8,615 indigenous crops meant for export to other countries were processed for quarantine clearance. Of these, 274 samples of different crops were found infested/ infected with various pests and 193 samples were salvaged by various physical and chemical treatments. However, 81 samples were rejected due to various pathogens. A total of 63 Phytosanitary Certificates were issued.
Seed health testing of germplasm for pest free conservation: A total of 1,14,988 indigenously collected or multiplied samples including 937 cryo preserved samples were processed, of which 11,942 samples were found infected with different pathogens and 981 samples were rejected due to heavy infection. A total of 479 samples were found contaminated with 36 types of weed seeds and all these samples were salvaged by mechanical cleaning. In addition, 809 samples meant for cryo-preservation were processed and found free from nematodes, weeds and insects but, 64 samples were infected with different fungi and all were salvaged.
Post entry quarantine growing/ monitoring: A total of 5,870 samples of exotic germplasm comprising Glycine max (1522), Phaseolus acuitifolius (2), P. coccineus (4), P. lunatus (54), P. vulgaris (1277), Pisum fulvum (1), P. sativum (266), Psophocarpus tetragonolobus (8), Vicia faba (367), V. sativa (10), Vigna spp. (76), V. radiata (700), V. trilobata (2), V. mungo (8), V. umbellata (102), V. unguiculata (1,436), V. unguiculata subsp. sesquipedalis (35) were grown in PEQ greenhouses at Headquarters. The seedlings were inspected for the presence of viral symptoms. The plants showing viral symptoms were carefully covered with muslin cloth bags. Leaf samples showing virus-like symptoms were tested for viruses using specific antisera to various seed-transmitted viruses by enzyme-linked immunosorbent assay (ELISA), electron microscopy and Reverse-transcription-PCR. A total of 90 post-entry quarantine inspections were carried out at various indenters’ sites. A total of 78,880 samples of various crops were inspected for presence of exotic pests (Table 1). The plants suspected to be infected by viruses of quarantine significance have been destroyed. The interceptions are presented in Table 1, 2. Beside this 124-post entry quarantine inspection of about 120000 samples for various crops were conducted at indenter’s sites.
Table 1. Exotic pests intercepted in germplasm imported into India during 2013-19 and are not reported from India
| Exotic Pest Intercepted |
Crop |
Source |
| Insects |
| Bruchus atomarius |
Lens culinaris |
Lebanon |
| B. ervi |
L. culinaris |
Lebanon, Syria |
| B. dentipes |
Vicia faba |
Lebanon |
| B. tristis |
L. culinaris |
Lebanon |
| Lathyrus sativus |
Lebanon |
| Carpophilus truncatus |
Zea mays |
USA |
| Fungi |
| Fusarium nivale |
Titicum aestivum |
UK |
| F. oxysporum f. sp. cucumerinum |
Cucumis sativus |
USA |
| Peronospora manshurica |
Glycine max |
Canada, Colombia, Japan, Taiwan |
| Phomopsis eres |
Robinia pseudoacacia |
Hungary |
| P. longicolla |
Helianthus annuus |
USA |
| Viruses |
| Bean mild mosaic virus |
Glycine max |
Canada, Columbia |
| Phaseolus vulgaris |
USA |
| Bean pod mottle virus |
G. max |
AVRDC (Taiwan) |
| P. lunatus |
AVRDC (Taiwan) |
| Vigna unguiculata |
Italy, IITA (Nigeria) |
| Broad bean mottle virus |
P. vulgaris |
AVRDC (Taiwan) |
| Broad bean stain virus |
G. max |
AVRDC (Taiwan), Costa Rica |
| V. radiata |
AVRDC (Taiwan) |
| V. unguiculata |
IITA (Nigeria) |
| Cherry leaf roll virus |
G. max |
AVRDC (Taiwan), Canada, Columbia, Costa Rica |
| V. unguiculata |
IITA (Nigeria), USA |
| Cowpea mottle virus |
P. vulgaris |
USA |
| V. faba |
Lebanon |
| V. sativa |
USA |
| V. unguiculata |
IITA (Nigeria) |
| Cowpea severe mosaic virus |
G. max |
AVRDC (Taiwan), Canada, Costa Rica |
| High plains virus |
Zea mays |
Thailand |
| Maize chlorotic mottle virus |
Z. mays |
Thailand |
| Pea enation mosaic virus |
G. max |
Costa Rica |
| V. unguiculata |
Italy |
| Peanut stunt virus |
G. max |
AVRDC (Taiwan), Canada, Costa Rica |
| V. sativa |
USA |
| Vigna radiata |
AVRDC (Taiwan), Costa Rica |
| V. unguiculata |
IITA (Nigeria), Italy |
| Pepino mosaic virus |
C. annuum |
Republic of Korea |
| S. lycopersicum |
Netherlands, Thailand, USA |
| Raspberry ringspot virus |
G. max |
AVRDC (Taiwan), Costa Rica, USA |
| V. unguiculata |
IITA (Nigeria) |
| V. unguiculata ssp. sesquipedalis |
Philippines |
| Tomato ringspot virus |
G. max |
AVRDC (Taiwan), Canada, Costa Rica |
| V. radiata |
AVRDC (Taiwan) |
| V. unguiculata |
Italy, IITA (Nigeria) |
| Weeds |
| Avena barbata |
Hordeum vulgare |
Morocco |
| Bifora testiculata |
H. vulgare |
Lebanon |
| Bromus diandrus |
Hordeum vulgare |
Morocco |
| Bromus secalinus |
Hordeum vulgare |
Morocco, USA |
| Cenchrus incertus |
Zea mays |
USA |
| Centaurea maculosa |
Coriandrum sativum |
Russia |
| Centaurea melitensis |
Hordeum vulgare, T. aestivum, |
Mexico, Morocco |
| Centaurea solstitialis |
Coriandrum sativum |
Russia |
| Cichorium pumilum Cichorium spinosum |
Trifolium alexandrinum |
Egypt, Uzbekistan |
| Convolvulus erubescens |
Hordeum vulgare |
Morocco |
| Echinochloa crus-pavonis |
Oryza sativa |
China |
| Echium plantagineum |
Hordeum vulgare |
Morocco |
| Fallopia convolvulus |
Hordeum vulgare, Lens culinaris |
Canada, Morocco |
| Galium aparine |
Hordeum vulgare |
Morocco |
| Galium boreale |
H. vulgare |
Lebanon |
| Galium tricornutum |
Lathyrus sativus, Lens culinaris, H. vulgare, Cucumis sativum |
Lebanon, Uzbekistan, Syria Arab Republic |
| Hordeum pusilum |
Lathyrus sativus |
Lebanon |
| Ipomoea plebeia |
Nigella sativa |
Uzbekistan |
| Lolium rigidum |
Hordeum vulgare |
Morocco |
| Lithospermum arvense |
Hordeum vulgare |
Morocco |
| Phalaris paradoxa |
Hordeum vulgare, T. aestivum, |
Lebanon, Mexico, Morocco |
| Polygonum arenastrum |
Hordeum vulgare |
Morocco |
| Polygonum cuspidatum |
H. vulgare, T. aestivum |
Morocco, Poland |
| P. lapathifolium |
O. sativa |
China |
| Raphanus raphanistrum |
Lens culinaris |
Australia |
| Salsola vermiculata |
Lens culinaris |
Canada |
| Scirpus debilis |
Oryza sativa |
Philippines |
| Sinapsis arvensis |
T. aestivum |
Lebanon |
Table 2. Pests not known to occur on the Host in India intercepted in germplasm imported during 2013-2019
| Pest Intercepted |
Crop |
Source |
| Fungus |
| Bipolaris maydis |
Capsicum annuum |
South Korea |
| Zea mays |
Mexico, Thailand, USA |
| Viruses |
| Arabis mosaic virus |
G. max |
AVRDC (Taiwan), Canada, Costa Rica |
| Phaseolus lunatus |
AVRDC (Taiwan) |
| Vigna radiata |
AVRDC (Taiwan) |
| V. unguiculata |
Italy, IITA (Nigeria) |
| Bean common mosaic virus |
P. acutifolius |
AVRDC (Taiwan) |
| P. lunatus |
AVRDC (Taiwan) |
| Broad bean wilt virus |
G. max |
Canada, Costa Rica |
| P. vulgaris |
USA |
| Grapevine fan leaf virus |
G. max |
AVRDC (Taiwan), Canada, Costa Rica, Nigeria, USA |
| V. radiata |
AVRDC (Taiwan) |
| V. unguiculata |
IITA (Nigeria) |
| Southern bean mosaic virus |
G. max |
AVRDC (Taiwan), Costa Rica |
| Tomato black ring virus |
G. max |
AVRDC (Taiwan), Canada, Costa Rica |
| V. unguiculata |
IITA (Nigeria) |
| Tobacco streak virus |
S. lycopersicum |
Netherlands, Thailand, USA |
| Nematode |
| Pratylenchus penetrans |
Malus domestica |
Netherland |
Besides, some regulated pests viz., 12 weeds, 08 viruses, 02 each of fungi and insects and 01 bacterium are also reported.
Supportive research
Thermal treatment against rice weevil: Thermal treatment i.e. 35, 40, 45, 50, 55 and 60±20C for a period of 1, 2 and 3 hours were evaluated against rice weevil, Sitophilus oryzae adult. Cent percent mortality of S. oryzae adult was observed at 45, 50, 55 and 60±1 0C for all time periods without affecting germination and vigour of wheat seeds.
DNA bar code Callosobruchus chinensis and C. maculatus: Cytochrome oxidase I gene based DNA barcode for C. chinensis (bp) 682 and C. maculatus (683) were developed.
Evaluation of non-edible oils against Callosobruchus chinensis: Hundred percent egg mortality was noticed in all non-edible oils of Pongamia glabra, Hydnocarpus wightiana, Madhuca longifolia, Callophyllum inophyllum, and Azadirachta indica compared to control. Similarly, all non-edible oils had ovipositional deterrence as compared to control. All non-edible oils caused hundred percent larval and adult mortality than control.
Loop-mediated isothermal amplification (LAMP) assay: Developed loop-mediated isothermal amplification (LAMP) assay for detection of Colletotrichum capsici targeting the ß-tubulin sequence with detection limit of 10 fg µl-1 of template DNA.
Species-specific PCR-based diagnosis of fungal/bacterial pathogens: Developed markers for species-specific PCR based diagnosis of fungal/bacterial pathogens such as Alternaria padwickii using TEF region, Bipolaris oryzae using ATCC 44560 unplaced genomic scaffold scaffold_136 region, Colletotrichum capsici using ß-tubulin region, Alternaria brassicae using ABC transporter gene region, A. brassicicola using SSR marker and Xanthomonas campestris pv. campestris using rpf gene region.
Multiplex PCR assay: Three sets of primers namely, Aba28sF and Aba28sR based on SSR marker for Alternaria brassicicola, ABC transporter (Atr1) gene-based primers, AbeABC1F and AbeABC1R for A. brassicae and rpf region-based primers namely rpfH_F and rpfH_R for Xanthomonas campestris pv. campestris were developed. The specific bands of 586 bp for A. brassicae, 201 bp for A. brassicicola and 304 bp for X. campestris pv. campestris were obtained in multiplex PCR. The sensitivity of the primer pairs is 100 pg µl-1 of template DNA.
Duplex PCR for simultaneous detection of Alternaria padwickii and Bipolaris oryzae infecting rice: A set of primers namely BoSP7-F and BoSP7-R designed from B. oryzae ATCC44560 unplaced genomic scaffold scaffold_136 whereas ApEF-1F and ApEF-1R were designed from elongation factor 1 region of A. padwickii. The specific bands of 175 bp for A. padwickii and 325 bp for B. oryzae were obtained in duplex PCR. The detection sensitivity of the primer pairs with the diluted genomic DNA revealed that it could detect up to 0.1 ng µl-1 of template DNA of both the pathogens.
Genetic diversity analysis of fungal pathogens using different molecular markers: Genetic diversity of fungal pathogens, namely, A. padwickii, Curvularia lunata and Fusarium spp. using SRAP markers, Bipolaris oryzae using URP and RAPD primers, B. sorghicola and C. capsici using URP and ISSR markers, C capsici and F. verticillioides using ISSR markers were analysed. Phylogenetic analysis among the isolates of fungal pathogens such as A. padwickii, Bipolaris spp., Curvularia spp., Exserohilum sp. using ITS markers and Fusarium spp. using ß-tubulin gene were done.
New host records of some fungal pathogens in indigenous plant genetic resources: New host records of some fungal pathogens in different crops for the first time from India and elsewhere were made such as Botrytis cinerea on Vernonia anthelmintica, Dinemasporium americana on Aegilops sp., Fusarium equiseti on Origanum vulgare, Lasiodiplodia theobromae on Costus speciosus, Myrothecium roridum on V. anthelmintica, Phompsis helianthi on Carthamus tinctorius, P. phaseoli on Oroxylum indicum and Dinemasporium americana on Aegilops sp.
Detached non-wounded fruit inoculation technique for pathogenicity of Colletotrichum capsici: Developed an inoculation technique in which spot inoculation with 20 µl conidial suspension (3-4 conidia/µl) of C. capsici in water gelatine (2.0 %) results in lesion development on detached non-wounded chilli fruits. This technique is suitable for virulence analysis of large number of isolates of C. capsici with a short period and could be utilized for screening of chilli genotypes for disease resistance.
Decade-long survival of fungal pathogens in conserved seeds of different crops: Reported survival of fungal pathogens for >10 years in cryo-preserved seeds of different crops such as Alternaria brassicicola in mustard, Colletotrichum capsici in chilli and Dendryphion penicillatum in opium.
Development of RT-PCR protocols for detection of viruses: Reverse-transcription - PCR protocols were developed for detection of Bean common mosaic necrosis virus and Peanut stunt virus (PSV). PSV is not reported from India.
Developed treatment for salvaging of nematode infested apple sapling: Root lesion nematode, Pratylenchus penetrans not reported from India was intercepted in apple (Malus domestica) rooted sapling imported from Netherlands. Root dip for 10 min in 0.25% and 0.3% formaldehyde solution eliminated nematode infection, however, root dip in 0.25% formaldehyde recommended, as it is cost effective.
Detected and identified nematode infection in Psoralea corylifolia: Medicinal herb, Psoralea corylifolia locally known as a babchi was found severely infestation with root-knot nematode, Meloidogyne incognita race-1.
Identified Acalypha indica as host of root knot nematode: Acalypha indica common weed in India was found to be infected with Meloidogyne incognita.
Survey of nematode infected pomegranate orchards: Pomegranate orchards in and around Jodhpur were found infected with root-knot nematode, Meloidogyne incognita. Therefore, the farmers were advised to use nematode free seedlings, to select nematode free area for pomegranate orchards while establishing the new orchards.
Survey for emerging nematode problem in guava orchards of Tamil Nadu: The survey was conducted in and around Coimbatore and Dindigul districts of Tamil Nadu for emerging root knot nematode infecting guava crop. Root-knot nematode species was found similar to quarantine nematode pest Meloidogyne enterlobii.
Identification of source of resistance to root-knot nematode in agri-horticultural crops: Identified several resistant sources against root-knot nematode, Meloidogyne incognita while screening of 2200 accessions of cultivated and wild germplasm of various crops.
Weed Risk Assessment (WRA) system developed
Weed Risk Assessment is a question based scoring system, containing 49 questions about the species. The questions include details of the plant’s climatic preferences, biological attributes, re-production and dispersal methods. The WRA uses responses to the questions to generate a numerical score that is positively correlated with weediness. The plants which have score between 0-6 are non weeds, 7-11 are common weeds and which have >12 score, are serious weeds.
Effect of wheat genotypes on the growth and development of weeds: The influence of wheat genotypes of different stature was studied on the growth and development of Phalaris minor, Chenopodium album and Melilotus indica was studied. The tall wheat genotype (plant height about 115 cm) exerted a strong suppressing effect on the weed intensity and height of these weeds.
Externally funded projects
Exploiting potential of electron beam irradiation as phytosanitary treatment against pulse beetles in seeds of selected grain legumes (BRNS, DAE): Electron beam (EB) irradiation has potential for disinfestation of seeds infested with different stages of Callasobruchus chinensis and C. maculatus. However, the mortality is caused at higher doses but sterility is caused at lower doses. Dose of 340 Gy at 500 Kev has been found an effective dose with no significant effect on seed quality parameters of black gram and cowpea. Dose dependent effects of irradiation were observed on insect and seed parameters. Sterility in adults is caused at a very low dose (25 Gy), however, adult mortality occurs at higher doses. Gamma irradiation with doses upto 200 Gy resulted in increase in vigour index in cowpea seeds.
Study of biological control of invasive plant species and Indian natural enemies (ICAR-CAB International)
Component B: Hedychium spp. complex (H. gardneriarum, H. flavescens, H. coronarium): Two surveys were undertaken in Sikkim areas and collected plant materials as well as infected/ infested samples were studied for their potential as natural enemies under controlled conditions at ICAR-NBPGR. Insect specimens submitted to National Pusa Collection at Division of Entomology, IARI for identification and pathogens submitted to Herbarium Cryptogamae Indiae Orientalis (HCIO) at Division of Plant Pathology, IARI and material sent to CABI, UK under MTA.
Race profiling and diversity analysis of Fusarium oxysporum f. sp. Lentis causing lentil wilt in India (DST Funded): Lentil wilt (Fusarium oxysporum f. sp. lentis (Fol)) is one of the important diseases worldwide including India. Two hundred and thirty-five isolates of the pathogen collected from different parts of India showed substantial variations in respect of morphological characters such as colony texture, pattern, pigmentation, growth rate, etc. The Fol populations (70 representative isolates) were grouped into 7 races on the basis of virulence pattern obtained on 10 differential genotypes and distribution pattern of races in the country was determined. The genetic diversity was analyzed using molecular markers namely, random amplified polymorphic DNA (RAPD), universal rice primers (URPs), inter simple sequence repeats (ISSR) and sequence related amplified polymorphism (SRAP). The populations from northern and central regions of India were appears distinct. The sequences of rDNA ITS and TEF-1a genes of the representative isolates were analyzed. Phylogenetic analysis indicating that the molecular groups partially correspond to the lentil growing regions of the isolates and races of the pathogen.
Development of DNA barcode and multiplex PCR based diagnostics for detection of nationally important seed borne fungal pathogens of major pulse crops for safe exchange and conservation (DBT Funded): Internal Transcribed Spacer (ITS), Translation elongation factor (TEF-a), ß-tubulin, Cytochrome c oxidase (COX), Large subunit of the rRNA (LSU) and Small subunit of the rRNA (SSU) genomic regions proved to be suitable genes for characterization as well as proved suitable for species identification of the pathogens namely, Rhizoctonia solani, Macrophomina phaseolina (R. bataticola), Ascochyta rabiei, Alternaria alternata, A. tunuissima, Fusarium oxysporum f. sp. ciceris, Sclerotium rolfsii, Sclerotinia sclerotiorum, Pseudocercospora cruenta and Cercospora canescens. Universal internal transcribed spacer (ITS) region based DNA barcodes were developed for identification of A. alternata, A. tenuissima, A. rabiei, F. oxysporum f. sp. ciceris, M. phaseolina, R. solani, S. sclerotiorum and C. canescens and submitted to the Barcode of Life Data System (BOLD).
The specific, highly sensitive and reliable conventional and real-time PCR assays were developed for diagnosis of the pathogens namely, Alternaria alternata, Rhizoctonia solani and Fusarium oxysporum f. sp. ciceris associated with the pulse crops. Universal internal transcribed spacer (ITS) region-based markers namely, BAA2aF & BAA2aR for A. alternata and BRS17cF & BRS17cR for R. solani were developed and amplified 400 bp and 200bp species specific amplicons, respectively. Whereas, COX II region based marker FOCox1F & FOCox3R was developed for F. oxysporum f. sp. ciceris with amplicon size of 150 bp. All the markers proved to be highly specific and sensitive and are able to detect up to 0.0001 ng template DNA using qPCR.
National containment/ quarantine facility for transgenic planting material (DBT-funded): With the approval of RCGM, 3,554 samples of imported transgenic planting material comprising Arabidopsis thaliana (64), Brassica napus (93), Eucalyptus sp. (1,585), Glycine max (8), Gossypium hirsutum (134), Manihot esculenta (512), Nicotiana tabacum (2), Oryza sativa (926) and Zea mays (230) were processed for quarantine clearance. Important pests intercepted include seven fungi and infected samples were salvaged by giving fungicidal treatment. Seed samples belonging to different crops were grown in the containment facility for ~45 days for detection of seed-transmitted pests not detectable in the laboratory tests. Also, 28 post-entry quarantine inspections of different crops were undertaken at the indenter’s site. Thirteen viruses were detected in corn and soybean including nine viruses not reported from India viz., Barley stripe mosaic virus, Bean mild mosaic virus, Cherry leaf roll virus, Cowpea severe mosaic virus, High plains virus, Maize chlorotic mottle virus, Raspberry ringspot virus, Tomato ringspot virus and Wheat streak mosaic virus and one virus Arabis mosaic virus is not known to occur on the host and with limited distribution in India.
All the imported transgenic lines were tested to ensure the absence of embryogenesis deactivator gene by PCR with primers specific to the cre-lox system. Plasmid cloned with cre sequence was used as positive control. In PCR amplification of cre sequence, the amplicon of 1031bp size was amplified only in positive plasmid sample while, no amplicon of corresponding size was observed in any of these transgenic samples ensuring the absence of embryogenesis deactivator gene. The samples were also tested for specific transgenic elements using PCR/ Real-time PCR based GM diagnostics.
A total of 58 samples comprising Arabidopsis thaliana (41) and Oryza sativa (17) were exported and two Phytosanitary Certificates were issued.
 |
|
Visual inspection of germplasm in Joint lab, Plant Quarantine Division, ICAR-NBPGR, New Delhi
|
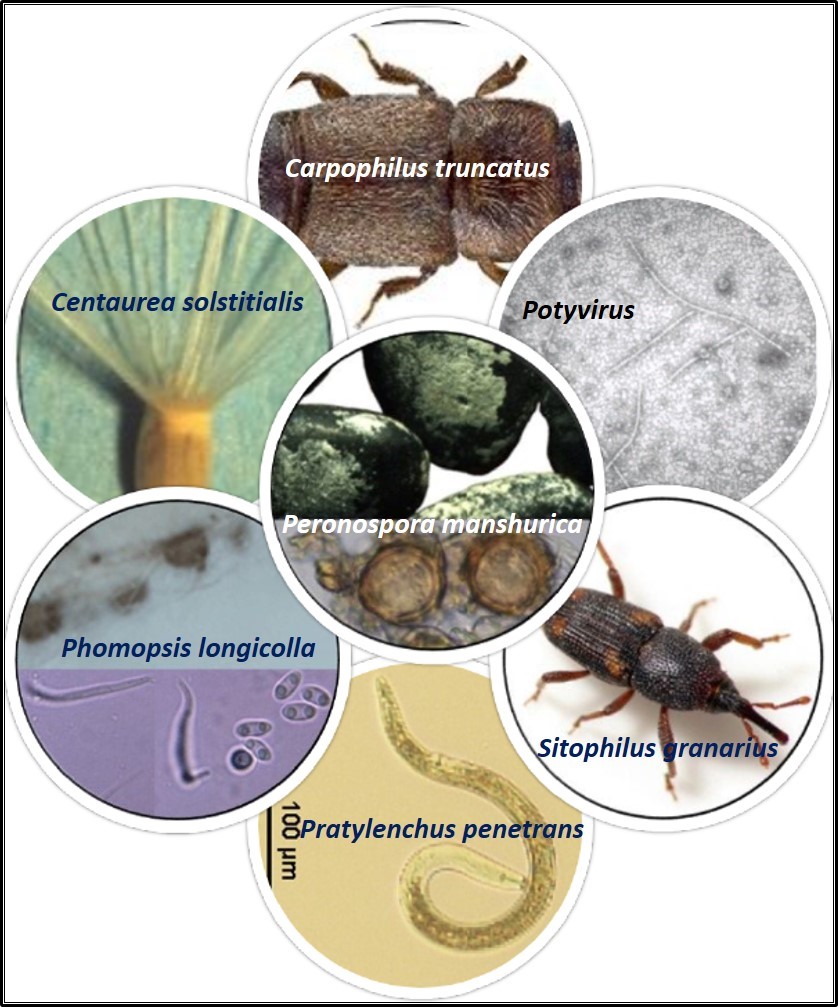 |
| Quarantine pests intercepted in imported germplasm. |
|
|
|
|
Adoption of user friendly Digitized Identification Keys for Bruchids by the end users in Directorate of Plant Protection, Quarantine and Storage and its 35 Plant Quarantine stations spread across the country and Entomologists across the world including those in various ICAR Institutes and SAUs for quick and reliable identification of bruchids of quarantine significance. The web version has >150 registered users within a span of 1 year of its upload.
|
|
|
- Dr SC Dubey awarded with J.F. Dasture Memorial Lecture Award 2015 of Indian Phytopathological Society, New Delhi.
- Dr S.C. Dubey elected as Fellow of National Academy of Biological Sciences (NABS), Chennai, India (2018)
- Dr V. Celia Chalam recognized as member, Potyviridae Study Group, International Committee on Taxonomy of Viruses (ICTV) during 2016-17.
- Dr V. Celia Chalam awarded Fellow of Indian Society of Plant Genetic Resources for outstanding contributions to the field of plant genetic resources in 2013.
- Dr V. Celia Chalam Awarded Fellow, Indian Phytopathological Society for outstanding contribution to the science of plant pathology for the year 2019.
- Dr Kavita Gupta awarded Fellow of Indian Society of Plant Genetic Resources for outstanding contributions to the field of plant genetic resources in 2014.
- Dr. MC Singh awarded Fellow of Society of Environment Sciences for outstanding contributions to the field of weed science in 2013.
- Dr. MC Singh was elected Member of National Academy of Sciences, India in 2015.
- Dr. MC Singh got third ICAR award in Inter-institutional competition of Hindi Essay writing in 2019.
- Dr. MC Singh was elected Councillor (North Zone) of Indian Society of Weed Science for 2019-2021.
- Dr Jameel Akhtar awarded 1st Prize under Hindi Kavita Path Pratiyogit’ on September 28, 2017 during Hindi Pakhwada-2017 organized by ICAR-NBPGR, New Delhi.
- Dr S.P. Singh received SSDAT Fellowship Award conferred by the Executive Committee of Society for Scientific Development in Agriculture and Technology, Meerut (U.P.) in 2014.
- Dr Pardeep Kumar conferred to Young Scientist Award by Astha Foundation during International conference on Global Research Initiatives for Sustainable Agriculture & Allied Sciences (GRISAAS-2017) at Maharana Pratap University of Agriculture and Technology, Udaipur, Rajasthan from December 02–04, 2017.
Best paper/ poster awards
- Outstanding poster award for the poster entitled Assessment of seed quality parameters in fruit-rot infected seeds of brinjal caused by Phomopsis vexans by Prasad Niranjan, A Kumar, J Akhtar, P Saha and SK Jha during 9th National Seed Congrees 2018-19 on Quality seed: a key component for doubling the farmer’s income organized by National Seed Research and Training Centre, Varanasi at Department of Genetics and Plant Breeding, Institute of Agricultural Science, BHU, Varanasi on Feb. 18-19, 2019.
- Best poster award for the poster entitled Simultaneous detection of dark leaf sport and black rot pathogen of crucifers by Raj Kiran, P Kumar, J Akhtar, B Singh, K Nair and SC Dubey presented during 1st International Conference on Climate Chnage and Adaptive Crop Protection for sustainable Agri-horticulture land scape organized by Society of Plant Protection Sciences, New Delhi held at ICAR-NRC on Seed Spices, Ajmer on Dec. 20-22, 2018.
- Second best poster award for the poster entitled Development of multiplex PCR for simultaneous detection of Alternaria brassicicola and Xanthomobnas campestris pv. campestris from brassica seed by Raj Kiran, P Kumar, J Akhtar, B Singh, K Nair and Sunil Chandra Dubey presented during a special National Symposium on Extension Plant Pathology: Technological Backstopping to the Farmers/Other Stakeholders organized by Indian Phytopathological Society at Indira Gandhi Krishi Vishwavidyalaya, Raipur on Sep. 25-26, 2018.
- Best Poster Award for the poster entitled “Application of weed Seeds in Imported Germplasm” by Ms Madhu Bala Priyadarshi, Singh MC, and Bhalla S during XIV National Seed Seminar, 2017, organized by Division of Seed Science Technology, ICAR-IARI, New Delhi-12 on 28-30 January 2017.
- Best poster award for the poster entitled Development of rapid diagnostic protocol for detection of Xanthomonas campestris pv. campestris causing black rot of crucifers using species-specific primers from rpf gene sequence by Raj Kiran, A Kandan, P Kumar, J Akhtar, B Singh and SC Dubey presented in National Symposium on Innovative strategies for the management of plant disease under climate change scenario organized by Indian Phytopathological Society (Delhi Zone) at Division of Plant Pathology, ICAR-IARI, New Delhi, India on Dec. 19th, 2017.
- Best poster award received for the paper entitled Development of bio-agent based module for integrated management of sheath blight of rice by Aradhika Tripathi, SC Dubey and SD Indira in the session of challenges in integrated crop management with respect to diseases during 6th IPS International Conference on Plants, Pathogens and People- Challenges in Plant Pathology to Benefit Humankind organized by Indian Phytopathological Society at NASC Complex, New Delhi, India from February 23-27, 2016.
- Best poster award for the poster entitled Pathogenic fungi not yet reported from India intercepted in imported germplasm by Jameel Akhtar, B Singh, A Kandan, P Kumar D Chand and SC Dubey presented during 6th IPS International Conference on Plants, Pathogens and People- Challenges in Plant Pathology to Benefit Humankind organized by Indian Phytopathological Society at NASC Complex, New Delhi, India from February 23-27, 2016.
- Third best poster award for the paper entitled Status of white tip nematode infection in rice germplasm meant for pest-free conservation in National Genebank by Gawade, BH, Z Khan, J Akhtar and SC Dubey in 6th IPS International Conference on Plants, Pathogens and People- challenges in plant pathology to benefit humankind, held at NASC complex, New Delhi, India, from 23-27 February, 2016.
- Third best poster award for the paper entitled Interception and salvaging of root-lesion nematode, Pratylenchus penetrans on apple (Malus domestica) saplings imported from The Netherlands by Khan Z, BH Gawade, DB Parakh and SC Dubey in 6th IPS International Conference on Plants, Pathogens and People- challenges in plant pathology to benefit humankind, held at NASC complex, New Delhi, India, from February 23-27, 2016.
- Best poster award received by Parakh DB, VC Chalam, D Ghosh, VK Baranwal, A Mishra, AK Maurya and N Verma. Post-entry quarantine procedures prevent entry of Brazilian citrus virus into India during the National Symposium on Biosecurity in Food Value Chain, at ICAR-National Bureau of Plant Genetic Resources, New Delhi, India on February 20, 2016.
- Second best poster award received by Chanda Priyadarshini, AK Maurya and VC Chalam. Detection of Bean yellow mosaic virus in French bean using EM, ELISA and RT-PCR during the National Symposium and Zone, IPS on Biosecurity in Food Value Chain at NBPGR, New Delhi on February 20, 2016.
- Smt Kavuri Sarada Memorial Award for the best research paper entitled “Genetic diversity analysis of Alternaria alternata isolates infecting different crops using URP and ISSR markers” by A Kandan, J Akhtar, B Singh, U Dev, R Goley, D Chand, A Roy, S Rajkumar and PC Agarwal (2014) published in Indian Journal of Plant Protection 42: 229-236.
- Best poster award for the paper entitlied Bioefficacy of electron beam irradiation against pulse beetle, Callosobruchus maculatus infesting black gram by Chauhan SK, PG Gore, R Pramod, S Gautam, J Dwivedi, TV Prasad, K Srinivasan and S Bhalla during National Symposium on Emerging Trends in Eco-friendly Insect Pest Management, held on January 22-24, 2014 at Department of Agricultural Entomology, Tamil Nadu Agricultural University (TNAU), Coimbatore.
- Second best poster paper presentation award for the poster paper entitled Reliable technique for screening of crucifers against mustard aphid, Lipaphis erysimi (Kalt.) under natural conditions and future perspectives by Singh SP, S Kumar, YP Singh and D Singh conferred by the Jury of 2nd National Brassica conference held at PAU. Ludhiana on February 14-16, 2014.
- >Best poster award for the poster entitled Rapid and sensitive detection of seed-borne pathogen Collectotrichum capsici in Capsicum annuum using loop-mediated isothermal amplification assay by Kandan A, J Akhtar, B Singh. D Pal, D Chand and PC Agarwal presented during 6th Indian Horticultural Congress organized by Indian Society of Horticulture, New Delhi, held at Coimbatore, India on November 6-9, 2014.
|
|
|
- Safe Transboundary Exchange of PGR
- Inspection of introduced germplasm including transgenics for detection of pests
- Identification of intercepted pests
- Post-entry quarantine growing and inspection
- Salvaging of infested/ infected/ contaminated germplasm
- Issue of Phytosanitary Certificate for material intended for export
- Pest-free Conservation of Germplasm
- Inspection of germplasm for detection of associated pests
- Salvaging of the infested/ infected/ contaminated germplasm without using pesticides
- Supportive Research
- Development of techniques for detection of various pests and salvaging of the infested/ infected germplasm
- Preparation of checklists of pests of various crops for risk analysis
- Assessment of prevalence of seed-transmitted viruses of legumes
- Screening of germplasm for pest resistance
- Policy Issues
- Technical input to ICAR and Department of Agriculture & Cooperation of Ministry of Agriculture on policy matters of quarantine including issues in EXIM, harmonization of phytosanitary standards with World Trade Organization (WTO) norms, etc.
- Human Resource Development
- Teaching of post graduate students
- Training courses on biosecurity, quarantine techniques for diagnostics of pests, biosafety and related policy issues
FACILITIES
There are well-equipped laboratories, trained scientists and standard procedures for a systematic and step-wise quarantine processing of germplasm for each discipline. The most important facilities include Transmission electron microscope, soft X-ray machine, vacuum fumigation system, multi-gas detector and ambient air analyzer, incubation and seed-storage rooms, hot water treatment tanks, UV spectrophotometer, ELISA reader, Real-time PCR, deep freezers, Real-time X ray Machine, ELISA reader, Ultracentrifuge, Vacuum fumigation plant etc.
Post-entry Quarantine Greenhouse Transmission Electron Microscope Real-time X ray Machine Real-time PCR
In addition, environmentally controlled glass/ screen house facilities for growing the germplasm suspected to carry viruses and germplasm treated with pesticides. A Containment Facility conforming to international standard of Containment Level-4 has also been established for quarantine processing of imported transgenic planting material.
|
CL-4 Containment/ Quarantine Facility for Transgenic Planting Material
|
- The facility of level 4 (CL-4) is meant for quarantine processing of imported transgenic planting material for research purposes
- The facility is completely sealed with air exchange through HEPA filters
- It has five bays with controlled conditions:
- air temperature 20-30ºC
- relative humidity 50-80%
- one of the bays designed for ground soil-based plants growing system
- Lab area for undertaking quarantine processing under contained conditions
|

(Inaugurated on October 25, 2001)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
27.
|
Wylie SJ, M Adams, VC Chalam, J Kreuze, JJ López-Moya, K Ohshima, S Praveen, F Rabenstein, D Stenger, A Wang, FM Zerbini and ICTV Consortium (2017). ICTV Virus Taxonomy Profile: Potyviridae.. J. General Virol., 98:: 352–354.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23.
|
Singh M, IS Bisht, M Dutta, K Kumar, L Kaur, A Sirari, Z Khan, AH Rizvi, A Sarker, KC Bansal (2014). Characterization and Evaluation of Wild Annual Cicer Species for Agro-morphological Traits and Major Biotic Stresses under North western Indian Conditions.. Crop Sci., 54:: 229-239.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inhouse Projects
|
| Programme | Project Title | Leader and Associates | Project Code |
|
1.1
|
Detection and Identification of Fungi and Bacteria in quarantine and Supportive Research
|
Dr Jameel Akhtar, Pardeep Kumar, Bharat Raj Meena, Raj Kiran and AK Maurya
|
PGR/DPQ-BUR-DEL-01.01
|
|
1.2
|
Detection and Identification of Viruses in Quarantine and Supportive Research
|
V. Celia Chalam, Puja Kumari and AK Maurya
|
PGR/DPQ-BUR-DEL-01.02
|
|
1.3
|
Detection and Identification of Insect and Mite Pests in Quarantine and Supportive Research
|
Dr Kavita Gupta, SP Singh, T. Boopathi (w.e.f. July 16, 2018) and DS Meena
|
PGR/DPQ-BUR-DEL-01.03
|
|
1.4
|
Detection and Identification of Nematode pests in quarantine and Supportive Research
|
Zakaullah Khan, Bharat H Gawade
|
PGR/DPQ-BUR-DEL-01.04
|
|
1.5
|
Detection and Identification of Intercepted Weeds in Quarantine and Supportive Research
|
Mool Chand Singh, DS Meena
|
PGR/DPQ-BUR-DEL-01.05
|
|
1.6
|
Quarantine treatments for disinfestation and disinfection of planting material against pests under exchange and supportive Research
|
Dr Bharat Hanamant Gawade, Meena Shekhar, Kavita Gupta, Z. Khan, Jameel Akhtar, T. Phoopathi, Pradeep Kumar, Raj Kiran, AK Maurya and DS Meena.
|
PGR/DPQ-BUR-DEL-01.06
|
|
1.7
|
Quarantine Processing of Imported Transgenic Germplasm and Supportive Research
|
V Celia Chalam, Kavita Gupta, Z. Khan, AK Maurya and DS Meena
|
PGR/DPQ-BUR-DEL-01.07
|
|
1.8
|
Seed health testing for conservation of indigenous germplasm free from pests
|
Pradeep Kumar, SC Dubey, V Celia Chalam, Meena Shekhar, Kavita Gupta, MC Singh, Z Khan, SP Singh, T. Bhoopathi, BH Gawade, Raj Kiran, Pooja Kumari, Veena Gupta, Sushil Pandey, AK Maurya, DS Meena and SL Jain
|
PGR/DPQ-BUR-DEL-01.08
|
|
|
|
Externally Funded Projects
|
| Programme | Project Title | Funding Agency | Principle Investigator | Date of Start | Date of Termination | Budget (Lakhs) | Project Code |
|
01
|
Race profiling and diversity analysis of Fusarium oxysporum f. sp. lentis causing lentil wilt in India
|
DST
|
SC Dubey
|
April 2014
|
March 2026
|
33.86
|
0000002
|
|
02
|
Development of DNA barcode and multiplex PCR based diagnostics for detection of nationally important seed borne fungal pathogens of major pulse crops for safe exchange and conservation.
|
DBT
|
S C Dubey
|
April 2018
|
March 2021
|
65.55
|
1009256
|
|
03
|
Mainstreaming of Sesame germplasm for productivity enhancement through genomics assisted core development and trait discovery (Subproject-3 Identification of Biotic Stress (Phyllody & Dry Root Rot) Tolerant Sesame Genotypes; Component-4 Dry Root Rot
|
DBT
|
SC Dubey
|
February 2020
|
February 2025
|
45.57
|
1012160
|
|
04
|
Mainstreaming of Sesame germplasm for productivity enhancement through genomics assisted core development and trait discovery Subproject-3 Identification of Biotic Stress (Phyllody & Dry Root Rot) Tolerant Sesame Genotypes; Component-1 Phyllody
|
DBT
|
V Celia Chalam
|
February 2020
|
February 2025
|
60.27
|
1012157
|
|
05
|
National containment/quarantine facility for transgenic planting material (component A)
|
DBT
|
V Celia Chalam
|
April 2013
|
March 2020
|
117.42
|
1001489
|
|
06
|
Leveraging genetic resources for accelerated genetic improvement of linseed using comprehensive genomics and phenotyping approaches (Sub-Project 4: Evaluation of linseed germplasm for major biotic stresses (Alternaria blight and Linseed bud fly), Component 6.)
|
DBT
|
T Boopathi
|
April 2020
|
March 2025
|
85.74
|
1012163
|
|
|
|
|
|
|
|
|
|
Dr. Celia Chalam Vasimalla, Head of Division
|
|
Division of Plant Quarantine
|
|
Phone: 91-9968299994
|
|
Email: celia.chalam(AT)icar.gov.in, mailcelia(AT)gmail.com
|
|
|
|
|
|
|
|
Dr. Jameel Akhtar, Principal Scientist
|
|
Division of Plant Quarantine
|
|
Phone: +919013718834
|
|
Email: jameel.akhtar(AT)icar.gov.in, jameelnbpgr(AT)gmail.com, jameelbau(AT)rediffmail.com
|
|
|
|
|
|
|
|
Dr. Kavita Gupta, Principal Scientist
|
|
Division of Plant Quarantine
|
|
Phone: 011-25802750; +91 9810349580
|
|
Email: Kavita.Gupta(AT)icar.gov.in, kavita6864(AT)gmail.com
|
|
|
|
|
|
|
|
Dr. Zakaullah Khan, Principal Scientist
|
|
Division of Plant Quarantine
|
|
Phone: 9013257919
|
|
Email: zakaullah.khan(AT)icar.gov.in, znema(AT)yahoo.com
|
|
|
|
|
|
|
|
Dr. Mool Chand Singh, Principal Scientist
|
|
Division of Plant Quarantine
|
|
Phone: +919958196700
|
|
Email: moolchand.singh(AT)icar.gov.in, mchsingh(AT)gmail.com
|
|
|
|
|
|
|
|
|
|
Dr. Pardeep Kumar, Scientist SS
|
|
Division of Plant Quarantine
|
|
Phone: +91-9466348907
|
|
Email: pardeep1(AT)icar.gov.in
|
|
|
|
|
|
|
|
Dr. Pooja Kumari, Scientist SS
|
|
Division of Plant Quarantine
|
|
Phone:
|
|
Email: , pooja.kumari(AT)icar.gov.in
|
|
|
|
|
|
|
|
Mr. Bharat Raj Meena, Scientist SS
|
|
Division of Plant Quarantine
|
|
Phone: +91 8459500734
|
|
Email: Bharat.Meena3(AT)icar.gov.in, brrm1406(AT)gmail.com
|
|
|
|
|
|
|
|
Ms. Raj Kiran, Scientist
|
|
Division of Plant Quarantine
|
|
Phone:
|
|
Email: raj.kiran(AT)icar.gov.in
|
|
|
|
|
|
|
|
Mr. Ashok Kumar Maurya, Chief Technical Officer
|
|
Division of Plant Quarantine
|
|
Phone: 9868131368
|
|
Email: ashok.maurya(AT)icar.gov.in, akmaurya368(AT)gmail.com
|
|
|
|
|
|
|
|
Dr. Dharm Singh Meena, Chief Technical Officer
|
|
Division of Plant Quarantine
|
|
Phone: 9971284904
|
|
Email: dharm.meena(AT)icar.gov.in, dsmeenapqd(AT)gmail.com
|
|
|
|
|
|
|
|
Dr. Smita Lenka, Assistant Chief Technical Officer
|
|
Division of Plant Quarantine
|
|
Phone: +91 9540646969
|
|
Email: Smita.Jain(AT)icar.gov.in
|
|
|
|
|
|
|
|
Dr. Sadhana Maurya, Technical Assistant
|
|
Division of Plant Quarantine
|
|
Phone: 9968868122
|
|
Email: Sadhna(AT)icar.gov.in, sadhnamry(AT)gmail.com
|
|
|
|
|
|
|
|
|
|
Mr. Satya Narayan Thakur, Skilled Supporting Staff SSS1
|
|
Division of Plant Quarantine
|
|
Phone: 9899410074
|
|
Email: Satnarayan.Thakur(AT)icar.gov.in
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|